How to Register Foreign Veterinary Products in Egypt


Contents Overview
Categories of Veterinary Product Registrations in Egypt

- Feed Additives or Nutritional Supplements
- Veterinary Medicines
- Veterinary Disinfectants
- Veterinary Vaccines
Registration Requirements for Veterinary Feed/Nutritional Supplements Registration in Egypt

While it is under the same guideline and general process as with Veterinary Pharmaceuticals, it often involves fewer technical document requirements. The detailed guideline of veterinary feed additive or nutritional supplements registration in Egypt can be found here: The process ensures that all products meet the nutritional, safety, and quality standards for animal consumption.
1. Dossier Submission:
The submission process begins with presenting a dossier containing the following:
- Product Composition: Detailed information about the ingredients, including active components, excipients, and their sources.
- Manufacturing Process: A comprehensive description of how the feed additive or supplement is produced, including quality control points and safety protocols.
- Nutritional Efficacy Data: Documentation supporting the efficacy of the product in improving animal health or nutrition.
- Safety Data: Studies and analyses proving the product’s safety for animal consumption, including any potential side effects or toxicity levels.
- Free Sale Certificate (FSC): Verifying the product is legally sold in its country of origin.
- Non-GMO Certificate: If applicable, this certificate ensures that the product does not contain genetically modified organisms.
Samples of the product must be submitted to the Central Laboratory for Animal Feed and Additives for thorough testing. The laboratory tests aim to verify that the product complies with Egyptian standards for animal feed, ensuring that it is safe and beneficial for animal consumption.
Tests conducted may include:
- Nutritional Analysis: Ensuring the product provides the claimed nutritional benefits.
- Contaminant Testing: Checking for the presence of harmful substances such as heavy metals, pesticides, or harmful microbial contaminants.
The MOA requires that all feed additives and supplements meet strict packaging and labeling standards. Labels must include:
- Composition Details: Listing all ingredients and their quantities.
- Instructions for Use: Clear guidance on how the product should be administered or mixed with animal feed.
- Safety Warnings: Any precautions or risks associated with the product's use.
- Nutritional Claims: The claims made regarding the benefits of the product must be substantiated with scientific evidence.
- Upon successful testing and dossier review, the MOA will issue a Registration Certificate for the feed additive or supplement, permitting its sale and distribution in Egypt.
- This certificate is typically valid for 5 to 10 years, depending on the type of product, after which renewal is required.
Estimated Timeline: The process generally takes 6 to 9 months, depending on the readiness of the dossier and the time required for laboratory testing.
SIMPLIFY REGISTRATION
Registration Requirements for Veterinary Medicine Registration in Egypt

The registration of veterinary medicines in Egypt follows a structured and multi-step process aimed at ensuring product safety, efficacy, and compliance with Good Manufacturing Practices (GMP). Below are the detailed steps:
1. Pre-Registration Requirements:
- The applicant (typically a manufacturer or local distributor) must have a registered account with the Egyptian Drug Authority (EDA).
- All manufacturing sites must be GMP-compliant and have the appropriate documentation to prove their compliance.
2. Dossier Submission:
- Site Master File (SMF): This document provides comprehensive information about the manufacturing facility, including personnel, equipment, layout, and quality control measures.
- Good Manufacturing Practice (GMP) Certificate: A valid certificate issued by a recognized authority confirming the manufacturing site meets GMP standards.
- Product Information: A detailed description of the product’s composition, active ingredients, excipients, and method of manufacture. This should also include Pharmacological Data, Stability Data, Clinical Efficacy, Toxicity Studies, and Quality Control Protocols.
- Certificates such as Free Sale Certificate (FSC) and Certificate of Pharmaceutical Product (CPP).
3. Scientific and Administrative Review:
- The dossier is reviewed by the scientific committee of the EDA. They will assess the product's safety, efficacy, quality control measures, and compliance with local and international standards.
- The process may include requests for additional information or clarification, depending on the product's complexity and dossier completeness.
4. Sample Testing and Analysis:
- Product samples must be submitted to an EDA-approved laboratory for analysis. The laboratory testing focuses on ensuring the product meets the specifications provided in the dossier, including the potency, purity, and stability of the product.
- Results from this analysis will contribute to the final decision on product approval.
5. Approval and Issuance of Registration Certificate:
- If the EDA is satisfied with the dossier and laboratory test results, they will issue a Veterinary Product Registration Certificate, allowing the product to be marketed and distributed in Egypt.
- The registration certificate is typically valid for 10 years, after which renewal is required.
Estimated Timeline: The entire process typically takes between 6 to 12 months, depending on the product’s complexity and the completeness of the initial dossier submission.
STREAMLINE REGISTRATION
Registration Requirements for Veterinary Disinfectant Registration in Egypt

This process ensures that all disinfectants meet safety and efficacy standards for animal and human health.The detailed guideline of veterinary disinfectant registration in Egypt can be found here: 1. Dossier Submission:
Required documents include:
- Product Composition: Detailed formula and active ingredients.
- Manufacturing Process: Full description of the production process.
The registration file must be uploaded to the EDA's online platform, accompanied by the required fees and documentation.
2. Labeling and Packaging:
Labels must comply with Egyptian standards, specifying usage instructions, safety warnings, and ingredient lists in Arabic and any other required languages.
3. Sample Testing:
Samples of the disinfectant must be submitted for laboratory testing to ensure they meet safety and efficacy standards. Disinfectants must prove effectiveness against targeted microorganisms and meet all regulatory requirements for veterinary environments.
4. Approval:
The application is reviewed by a specialized committee. Upon successful evaluation, the product is granted a Registration License, valid for 10 years.
5. Preliminary Approval for New Products:
A preliminary approval is issued for new products, which allows manufacturers to produce and test batches locally under controlled conditions. For imported products, stability studies and product samples must be submitted for verification.
6. Renewal and Extension:
Products must be re-registered before the 10-year expiration.Re-registration is required every 10 years, with additional stability data and potential sample testing during re-registration. If additional time is needed to complete requirements, an extension can be granted.
Key Considerations for Disinfectants Registration
- Toxicity Data: Disinfectants must provide comprehensive toxicity reports to demonstrate safe usage in veterinary environments.
- Environmental Impact: If applicable, the product’s potential effects on the environment, such as residual toxicity, must be evaluated.
Registration Requirements for Veterinary Vaccines Registration in Egypt
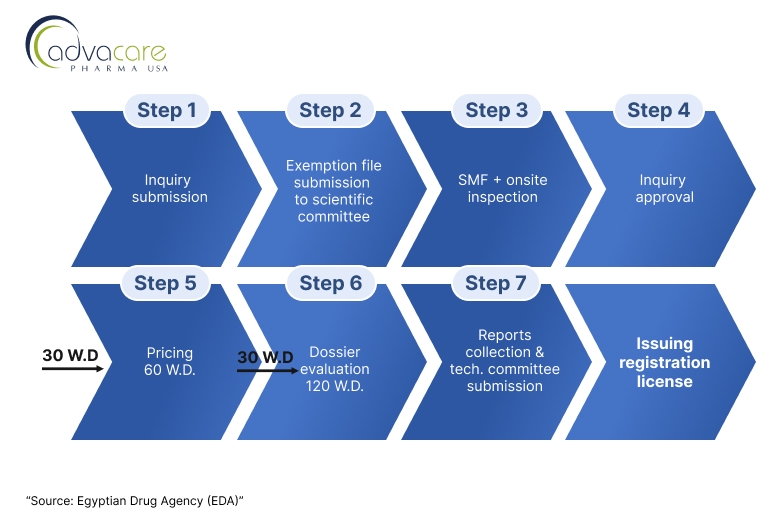
Distributors looking to register foreign veterinary vaccine finished products, particularly from non-reference country origins can follow the steps laid under Process B of the article summarized by the following flowchart:
1. Application Inquiry Submission
The applicant submits an application inquiry for inquiry approval, the applicant 201 will be asked to contact the scientific file examination unit to submit an exemption file within 20 working days or inquiry will be canceled.
2. Site Documents Submission and Onsite Inspection:
After the approval of the scientific specialized committee for biological products, the applicant should submit the site master file (SMF) to be evaluated by the biological inspection department. In case of approval of the submitted SMF, the inspection department shall inspect the site for compliance with GMP. Inspectors will assess whether the site adheres to GMP standards and local Egyptian regulations.
3. Dossier Submission:
Upon approval of the manufacturing site GMP compliance, the applicant must submit a comprehensive dossier. The dossier must include:
- Product Composition: Detailed information on the vaccine's active and inactive ingredients.
- Manufacturing Process: A clear outline of the manufacturing process, including details on raw materials and quality control measures.
- Stability Data: Stability studies proving that the vaccine maintains its efficacy throughout its shelf life.
- Safety and Efficacy Data: Results from clinical trials, often including local studies to prove that the vaccine is effective and safe in Egypt’s animal populations.
- GMP Certification: Certification from the vaccine’s manufacturing site proving compliance with Good Manufacturing Practices (GMP).
- Free Sale Certificate (FSC) or equivalent, showing that the vaccine is approved for sale in its country of origin.
- Vaccine samples must be submitted to an approved laboratory for quality control testing. The tests typically verify the vaccine's potency, safety, and compliance with the dossier specifications.
- Vaccines may undergo local efficacy trials if required by the MOA to demonstrate their effectiveness in local conditions.
- The labeling and packaging must comply with Egyptian regulations. This includes ensuring the label contains the proper instructions, warnings, and batch information in Arabic and any other required languages.
- Upon successful completion of the dossier review and laboratory testing, GAVS will issue a Vaccine Registration Certificate, allowing the vaccine to be marketed and sold in Egypt.
- The registration certificate typically needs to be renewed periodically, with renewal often requiring updated stability data and possibly new sample testing.
- The process can take 9 to 12 months, depending on the complexity of the vaccine, the completeness of the dossier, and any required testing or site inspections.
FAST-TRACK REGISTRATION
Simplifying the Registration Process with AdvaCare Pharma

Going through the complex process of registering foreign veterinary products in Egypt requires a deep understanding of each country’s specific regulations, from dossier submission to local testing and post-market surveillance. Partnering with an experienced company like AdvaCare Pharma, which has extensive expertise in the region, ensures a smoother and more efficient registration process. With our insights and tailored services, we help you overcome regulatory challenges and successfully register your veterinary products across the Middle East.
It is important for distributors to work with manufacturers that are able to provide utmost technical support in this highly complex process.
How Do We Make Registrations Easier?
- Streamlined Document Preparation and Authentication AdvaCare Pharma ensures that all site documents, including the Site Master File (SMF) and GMP certificates, are constantly up-to-date and ready for submission. This allows for a fast start in the site registration process. We prioritize the authentication process and, with the distributor's confirmation, make sure that the correct documents are legalized right away, minimizing delays and avoiding unnecessary costs due to the wrong documents being processed.
- Experienced in GMP Review and Site Inspections AdvaCare Pharma has successfully passed multiple GMP reviews and site inspections across the region, meeting the high standards expected by local authorities. Our production facilities maintain stringent quality control measures, ensuring compliance with regulatory requirements. From facilitating visa applications to guiding inspectors during site visits, AdvaCare Pharma efficiently manages the entire inspection process, providing a seamless experience for distributors and inspectors alike.
- Comprehensive and High-Quality Dossier Submission At AdvaCare Pharma, we prioritize quality and compliance in every dossier submission. Our experienced technical team ensures that each dossier is tailored to meet the specific requirements of the country where the registration is being filed. By thoroughly gathering all necessary internal and external references before starting the registration process, we minimize the chances of additional requests or rejections from local authorities, making the review process smoother and faster.
- Custom Packaging and Sample Quality Packaging is a critical element in product registration, and AdvaCare Pharma goes the extra mile to ensure it meets local standards. We collaborate closely with our distributors to design and produce packaging artwork that complies with local regulations. Our samples are manufactured according to registration requirements to ensure the highest quality, which aligns with each country’s stringent testing and evaluation requirements. This approach guarantees that our products meet all local standards, facilitating a successful and efficient registration process.
AdvaCare Pharma understands the complexities of navigating the rigorous registration requirements for veterinary products. With several products already registered in the GCC region and 65+ countries worldwide, we provide a streamlined and reliable path to market success. Our commitment to regulatory excellence, combined with tailored support and packaging solutions, ensures that your registration process is smooth and efficient.
Join our global network of trusted distributors today. As an AdvaCare Pharma partner, distributors will benefit from comprehensive regulatory and technical assistance, ensuring faster market access for high-quality, affordable products. We also offer exclusive distributor perks, including comprehensive marketing support, product training, and sales resources to help grow your business. With a commitment to strong partnerships, we are dedicated to your long-term success. Visit our website and apply for distributorship to gain a competitive edge in your region.
References: EDA 2024 "EDA" EDA 2023 "Guidelines on Registration Procedures of Veterinary Pharmaceuticals" EDA 2023 "Regulatory and Guidance Guideline and Business Rules for Antiseptics/Disinfectants Registration" EDA 2023 "Guideline for Registration of Biosimilar Products in Egypt" MEVMAS 2024 "Middle East Veterinaty Medicine and Animal Science Conference"
Don't want to miss the next AdvaCare article?

Recommended Content

Leveraging Pharma Expertise to Thrive in Supplement Distribution


Ecuador: Importing Medicines with an International Pharmaceutical Manufacturer


How Distributors Can Win With Multi-Format Supplement Portfolios Aligned to Global Trends
