- Home›
- Pharmaceuticals›
- Pharmaceutical Tablets›
- Cold Relief Tablets
Cold Relief Tablets
Dosage
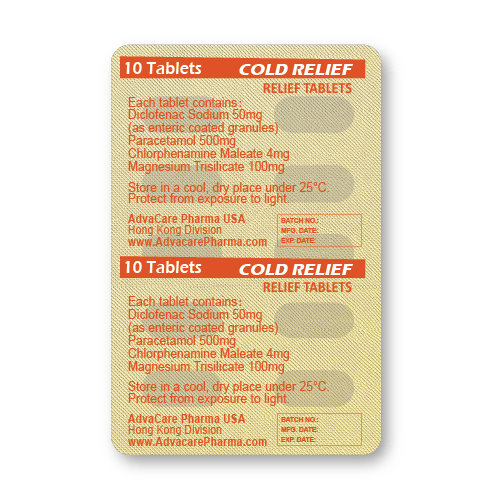
Packaging
What are Cold Relief Tablets?
Active Ingredients: Paracetamol, Diclofenac Sodium, Chlorpheniramine Maleate, Magnesium Trisilicate
Cold Relief Tablets contain paracetamol + diclofenac sodium + chlorpheniramine maleate + magnesium trisilicate. This fixed-dose combination drug is used to temporarily relieve symptoms associated with the common cold, such as cough, watery eyes, fever, pain, itchy eyes, runny nose, and sneezing.
Cold Relief Tablets contain four active ingredients:
- Paracetamol (acetaminophen) is a commonly used painkiller and antipyretic. It reduces fever and relieves mild to moderate pain relief.
- Chlorpheniramine maleate is an antihistamine. It works by blocking histamine that the body makes during an allergic reaction.
- Diclofenac sodium is a non-steroidal anti-inflammatory drug (NSAID). It helps to reduce substances in the body that cause pain and inflammation.
- Magnesium trisilicate is used as an antacid. It can help relieve pain and discomfort caused by indigestion and heartburn.
Cold Relief Tablets are produced with a fixed dosage of 500mg paracetamol, 50mg diclofenac sodium, 4mg chlorpheniramine maleate, and 100mg magnesium trisilicate. These tablets are packaged on blisters and available for distribution as boxes of 10 tablets or 100 tablets.
AdvaCare Pharma is a trusted global supplier of Cold Relief Tablets. This medication is manufactured in our factories in China, India, and the USA. We routinely inspect our manufacturing facilities to ensure our products meet quality and safety standards.
Why are we a leading Cold Relief Tablets manufacturer?
As a reputable Cold Relief Tablets manufacturer, we are dedicated to ensuring that GMP guidelines and standards strictly apply to the manufacture of our entire range of 200+ pharmaceutical treatments in tablet dosage form.
AdvaCare Pharma is an American pharmaceutical company committed to the manufacture of high-quality, affordable pharmaceuticals for a global market. The extensive international network that we partner with includes pharmaceutical distributors, hospitals, pharmacies, and a variety of other medical institutions. Our vision is to manufacture Cold Relief Tablets, and other quality-assured oral solid treatments, that get into the hands of those that need them most.
Uses
What is Paracetamol + Diclofenac Sodium + Chlorpheniramine Maleate + Magnesium Trisilicate used for?
It is used to relieve symptoms caused by the common cold or other ailments. It can help reduce fever, headache, body ache, toothache, neck pain, or arthritic pain. It can also reduce stuffy nose, watery or itchy eyes, and sneezing.
How should Cold Relief Tablets be used?
This medication is intended to be taken orally. It is recommended to take the tablets with food to prevent an upset stomach. The tablets should be swallowed whole and should not be crushed or split. Do not take Cold Relief Tablets for longer than recommended.
How should Cold Relief Tablets be stored?
These tablets should be kept in their original packaging until use. It is recommended to keep them in a dry location that is below 30°C.
What dose should be taken?
Adult Dosing The usual dose is 1 tablet taken every 6 hours.
The dosage is based on medical condition, response to treatment, age, and weight. Refer to a doctor or pharmacist for guidelines on dosage. Do not exceed what they advise.
What if a dose of Cold Relief Tablets is missed?
The missed dose should be taken as soon as possible. If it is nearly time for the next scheduled, the missed dose should be skipped. Never take 2 doses at the same time.
Who can use Cold Relief Tablets?
Cold Relief Tablets can be given to adults, but caution is advised for specific groups of patients.
Pregnant This medicine should be avoided by women who are pregnant. It is important to note that combination drugs with Diclofenac Sodium should only be used if the benefits outweigh the risks in women who are less than 30 weeks gestation. This is particularly important during 20-29 weeks’ gestation. After 30 weeks, Diclofenac Sodium should be avoided. At weeks 20-29 gestation, the risk of embryo-fetal toxicity and death is low. The risk of fetal harm, such as renal dysfunction and oligohydramnios, starts around week 20 gestation. Based on human data with NSAIDs premature ductus arteriosus closure begins at 30 weeks gestation.
Combination drugs containing Magnesium Trisilicate should be avoided during pregnancy, as the prolonged use or high-dose therapy of this drug has been associated with fetal harm, including nephrolithiasis and hypotonia.
Women of Reproductive Potential Combination medicines containing Diclofenac Sodium should be avoided for female patients who are trying to conceive.
Breastfeeding This medicine should only be used if the benefits outweigh the risks, as the safety and efficacy of this combination medication have not been established. In regard to Chlorpheniramine, there is a possible risk of infant depression based on limited human data with other antihistamines, and a theoretical risk of decreased milk production.
Children Appropriate studies have not been performed to assess the safety and efficacy of this fixed-dose medication in children.
Geriatric Combination medicines containing Diclofenac should be used cautiously in elderly patients. Diclofenac is associated with stomach or intestinal bleeding, and this population is at an increased risk of adverse effects.
Other warnings
For patients with renal disease, this medicine should be used cautiously. As this is a fixed-dose medication, it may not be possible to adjust the dose. This medication should not be given to those with severe or active liver disease.
NSAIDs are associated with an increased risk of serious thrombotic events, including myocardial infarction and stroke. The risk of this increases with the length of use.
NSAIDs can increase the risk of severe gastrointestinal effects, such as bleeding, ulceration, or intestinal perforation. There have been reports of fatal outcomes.
Side Effects
As with all pharmaceuticals, some unwanted effects can occur from the use of Cold Relief Tablets.
Common side effects include, but may not be limited to:
- drowsiness
- dizziness
- upset stomach
- nausea or vomiting
- blurry vision
- drowsiness
- indigestion
- loss of appetite
- gas
- increased blood pressure
- swelling or pain in arms or legs
Stop using this medicine and call a doctor if the following symptoms develop:
- skin rash
- signs of a heart problem, such as swelling, feeling short of breath, or rapid weight gain
- signs of a kidney problem
- signs of a liver problem, such as dark urine, clay-colored stools, or jaundice
- signs of stomach bleeding, such as bloody or tarry stools or coughing up blood
It is advised to seek immediate medical help in the event of:
- allergic reaction
- drug reaction
- signs of heart attack or stroke
For a comprehensive understanding of all potential side effects, consult a medical professional.
If any symptoms persist or worsen, or you notice any other symptoms, please call your doctor immediately.
Precautions
Do NOT use Cold Relief Tablets if:
- You are allergic or hypersensitive to any of the ingredients.
- You have had an asthma attack or severe reaction to aspirin or another NSAID (diclofenac sodium).
- You have recently had or have an upcoming heart bypass surgery (diclofenac sodium).
- You have severe active hepatic disease (paracetamol).
- You have acute asthma or sleep apnea (chlorpheniramine).
- You are taking or have taken an MAOI in the past 14 days.
Due to possible drug interactions, consult with your doctor about any medications, supplements, or herbal products that you are taking before beginning your treatment with Cold Relief Tablets. The ingredients in this medication may have possible interactions with antipsychotics, antiplatelet (e.g. aspirin), anticoagulants (e.g. warfarin), corticosteroids, SSRIs, barbiturates, hypnotics, opioid analgesics, sedatives, MAO inhibitors, beta-blockers, ACE inhibitors, other NSAIDs, baclofen, or pemetrexed. This is not a complete list of possible interactions.
This medication may not be suitable for people with certain conditions, so it is important to consult with a doctor if you have any health conditions. Special considerations or additional monitoring may be necessary for conditions such as:
- bronchospasm
- cardiovascular disease
- hepatic porphyria
- hypertension
- fluid retention
- renal impairment
- hepatic impairment
- G6PD deficiency
- hypophosphatemia
- epilepsy
- thyroid problems
- systemic lupus erythematosus
- narrow-angle glaucoma
- prostatic hypertrophy
- ulcer or pyloroduodenal obstruction
- smoking
- alcohol dependence or drinking 3 or more alcoholic beverages per day
- elderly
- children
- pregnant or breastfeeding
Combination medicines containing diclofenac can affect ovulation. It may be harder to get pregnant while you are using this medicine.
Cold Relief Tablets may cause side effects such as drowsiness, dizziness, or blurred vision. It is not advised to drive or operate machinery while taking this medication.
It is advised to avoid drinking alcohol while being treated with this medicine.
Ingredients in this medication may interfere with the results of certain types of medical tests, such as skin antigen tests. It is important to inform laboratory staff if you have taken this drug.
Medications containing paracetamol may interfere with the results of some types of blood glucose monitors.
It is recommended to avoid becoming overheated or dehydrated, such as while exercising or in hot weather. Ingredients, such as chlorpheniramine, may decrease the body's ability to sweat, which can increase the risk of heat stroke.
Do not take other pain medications or cold/flu medicines while taking Cold Relief Tablets.
Do not take more than the recommended dose of this medication. Paracetamol is associated with liver failure when used at higher than prescribed doses.
References
Acetaminophen (paracetamol) for the common cold in adults
This study’s main goal was to determine the efficacy and safety of acetaminophen in the treatment of the common cold in adults. It included randomized controlled trials (RCTs) comparing acetaminophen to placebo or no treatment in adults with the common cold.
The following primary outcomes were measured: symptom score and duration of common cold symptoms, as well as secondary outcomes like overall well-being, adverse events, and financial costs.
This trial included 758 participants or 4 trials. The results showed that patients treated with paracetamol had significant improvements in nasal obstructions in 2 of the 4 selected studies. One study also showed that acetaminophen is superior to placebo in decreasing rhinorrhoea severity. Two studies revealed that headache and achiness improved more in the acetaminophen group than in the placebo group.
The conclusion of this study is that acetaminophen can relieve nasal obstruction and rhinorrhoea. This drug is commonly used in cases of the common cold in adults.

You might be interested in...
Why AdvaCare Pharma?
As an industry leader, we are aware of our responsibility to provide affordable and sustainable solutions to improve healthcare worldwide.